2017年上半年FDA批准新药大盘点
时间:2017-08-03 来源:成都先导
在2017年前6个月里,美国FDA共批准了23个新药(详情如下),超过了2016年全年批准的数量。根据分子实体类型来看,前6个月批准了12个小分子药物(约占52%),名副其实地占据了半壁江山(详情见表1)。
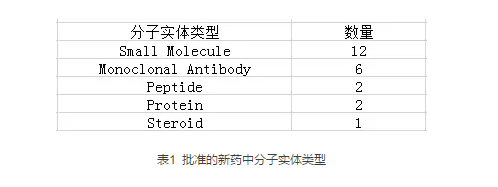
根据给药途径来看,23种新药中有13种可以通过口服给药(Zejula既可以口服也可以静脉注射),7种新药能够通过静脉注射给药,4种能够通过皮下给药方式。(详情见表2)
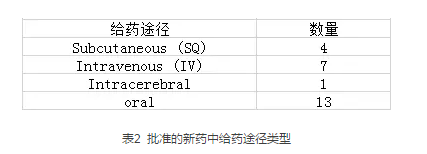
下表展示了这批新药所对应的研究靶点,里面包括前段时间火热的Teva的氘代药物AUSTEDO™,它也是第一个FDA批准的氘代药物。其对应的研究靶点是Vesicular monamine transporters (VMATs),而针对该靶点Neurocrine Biosciences的Ingrezza也在上半年获批。

2017年前6个月FDA批准的新药详情
1. Plecanatide (Trulance)
药物名称: Plecanatide (Trulance)
公司: Synergy Pharmaceuticals
靶点: Guanylyl cyclase c Receptor
分子类型: Peptide
给药途径: Oral (PO)
适应症: Chronic idiopathic constipation(Approval)
Irritable Bowel Syndrome (IBS) (NDA)
推荐给药剂量:3 mg taken orally once daily.
2. Etelcalcetide (Parsabiv)
药物名称: Etelcalcetide (Parsabiv)
公司: Amgen
靶点: Protein Kinase C (PKC)
分子类型: Peptide
给药途径: Intravenous (IV)
适应症: Secondary hyperparathyroidism in patients with chronic kidney disease on haemodialysis
推荐给药剂量:5 mg administered by intravenous (IV) bolus injection three times per week at the end of hemodialysis treatment.
3. Deflazacort (Emflaza)
药物名称: Deflazacort (Emflaza)
公司: PTC Therapeutics
靶点: Glucocorticoid Receptor (GR)
分子类型: Steroid
给药途径: Oral (PO)
适应症: Duchenne Muscular Dystrophy (DMD)
推荐给药剂量:approximately 0.9 mg/kg/day once daily.
4. Brodalumab (Siliq)
药物名称: Brodalumab (Siliq)
公司: Valeant
靶点: IL-17 Receptor (IL-17R)
分子类型: Monoclonal Antibody
给药途径: Intravenous (IV), Subcutaneous (SQ)
适应症:
Psoriasis(Approved);
Psoriatic Arthritis (PA)( Approved in other than U.S./E.U.);
Axial Spondyloarthritis(Development Outside U.S.);
推荐给药剂量:210 mg administered by subcutaneous injection at Weeks 0, 1, and 2 followed by 210 mg every 2 weeks.
5.Telotristat etiprate (Xermelo)
药物名称: Telotristat etiprate (Xermelo)
公司: Lexicon Pharmaceuticals
靶点: Tryptophan hydroxylase
分子类型: Small Molecule
给药途径: Oral (PO)
适应症: Neuroendocrine Tumors (NET);
Crohn's Disease(Suspended);
Ulcerative Colitis (UC) (Suspended);
推荐给药剂量: 250 mg three times daily for patients whose diarrhea is inadequately controlled by SSA therapy in adult patients.
6.Avelumab (Bavencio)
药物名称: Avelumab (Bavencio)
公司: Merck KGaA
靶点: Immune system Programmed death-1 receptor (PD-1) / Programmed death ligands (PD-L1 and PD-L2)
分子类型: Monoclonal Antibody
给药途径: Intravenous (IV)
适应症:
Merkel Cell Carcinoma(Approved);
Bladder Cancer(Approved);
Renal Cell Cancer (RCC)(PhIII);
Diffuse Large B-Cell Lymphoma (DLBCL) – NHL(PhIII);
Head and Neck Cancer(PhIII);
Non-Small Cell Lung Cancer (NSCLC)(PhIII);
Ovarian Cancer(PhIII);
Gastric Cancer(PhIII);
Solid Tumors(PhI/II);
Hodgkin's Lymphoma(PhI).
推荐给药剂量: 10 mg/kg administered as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity.
7. Ocrelizumab (Ocrevus)
药物名称: Ocrelizumab (Ocrevus)
公司: Genentech/Roche
靶点: Cluster of Differentiation 20 (CD20)
分子类型: Monoclonal Antibody
给药途径: Intravenous (IV)
适应症: Relapsing and primary progressive forms of multiple sclerosis
推荐给药剂量:
8. Dupilumab (Dupixent)
药物名称: Dupilumab (Dupixent)
公司: Regeneron
靶点: IL-4 Receptor (IL-4R)
分子类型: Monoclonal Antibody
给药途径: Subcutaneous (SQ)
适应症:
Atopic Dermatitis (Eczema)(Approved);
Asthma(PhIII);
Nasal Polyposis(PhIII);
Esophagitis(PhiI);
Food Allergies(Preclinical).
推荐给药剂量: an initial dose of 600 mg (two
300 mg injections), followed by 300 mg given every other week for adult patients.
9. Ribociclib (Kisqali)
药物名称: Ribociclib (Kisqali)
公司: Novartis
靶点: Cyclin Dependent Kinase (CDK)
分子类型: Small Molecule
给药途径: Oral (PO)
适应症: HR-positive, HER2-negative breast cancer
推荐给药剂量: 600 mg (three 200 mg film-coated tablets) taken orally, once daily for 21 consecutive days followed by 7 days off treatment resulting in a complete cycle of 28 days. KISQALI can be taken with or without food.
10. Safinamide (Xadago)
药物名称: Safinamide (Xadago)
公司: US WorldMeds
靶点: Calcium Channel Monoamine oxidase B (MAO-B) Dopamine Reuptake Sodium Channels
分子类型: Small Molecule
给药途径: Oral (PO)
适应症: Parkinson's Disease (PD)
推荐给药剂量: 50 mg administered orally once daily (at the same time of day), without regard to meals. After two weeks, the dosage may be increased to 100 mg once daily, based on individual need and tolerability. Daily dosages of XADAGO above 100 mg have not been shown to provide additional benefit, and higher dosages increase the risk for adverse reactions. XADAGO has been shown to be effective only in combination with levodopa/carbidopa [see Indications and Usage (1)]. If a dose is missed, the next dose should be taken at the same time the next day. XADAGO 100 mg should be tapered by decreasing the dose to 50 mg for one week before stopping
11. Naldemedine (Symproic)
药物名称: Naldemedine (Symproic)
公司: Shionogi
靶点: Opioid receptors
分子类型: Small Molecule
给药途径: Oral (PO)
适应症: Opioid Induced Constipation (OIC)
推荐给药剂量: 0.2 mg orally once daily with or without food for adult.
12. Niraparib (Zejula)
药物名称: Niraparib (Zejula)
公司: Tesaro
靶点: Poly ADP-Ribose Polymerase (PARP)
分子类型: Small Molecule
给药途径: Oral (PO)
适应症:
Ovarian Cancer(Approved);
Breast Cancer(PhIII);
Prostate Cancer(PhII);
Bone Cancer(PhI).
推荐给药剂量: 300 mg (three 100 mg capsules) taken orally once daily as monotherapy.
13. Cerliponase alfa (Brineura)
药物名称: Cerliponase alfa (Brineura)
公司: BioMarin
靶点: Tripeptidyl Peptidase-1 (TTP1)
分子类型: Protein
给药途径: Intracerebral
适应症: Neuronal Ceroid Lipofuscinosis (NCL)
推荐给药剂量: in pediatric patients 3 years of age and older is 300 mg administered once every other week by intraventricular infusion。
14. Midostaurin (Rydapt)
药物名称: Midostaurin (Rydapt)
公司: Novartis
靶点:
FMS-like tyrosine kinase 3 (FLT-3)
VEGF Receptor (VEGFR)
Platelet-derived growth factor receptor (PDGFR)
Protein Kinase C (PKC)
KIT/c-KIT
分子类型: Small Molecule
给药途径: Oral (PO)
适应症:
Acute Myelogenous Leukemia (AML);
Mastocytosis.
推荐给药剂量: for patients with acute myeloid leukemia is 50 mg orally twice daily with food on Days 8 to 21 of each cycle of induction with cytarabine and daunorubicin and on Days 8 to 21 of each cycle of consolidation with high-dose cytarabine
15. Deutetrabenazine (Austedo)
药物名称: Deutetrabenazine (Austedo)
公司: Teva
靶点: Vesicular monamine transporters (VMATs)
分子类型: Small Molecule
给药途径: Oral (PO)
适应症:
Huntington's Disease(Approved);
Tardive Dyskinesia(NDA);
Tourette's Syndrome(PhI).
推荐给药剂量:The starting dose of AUSTEDO is 6 mg administered orally once daily. The dose of AUSTEDO may be increased at weekly intervals in increments of 6 mg per day to a maximum recommended daily dosage of 48 mg. Administer total daily dosages of 12 mg or above in two divided doses. Administer AUSTEDO with food.
16. Valbenazine (Ingrezza)
药物名称: Valbenazine (Ingrezza)
公司: Neurocrine Biosciences
靶点: Vesicular monamine transporters (VMATs)
分子类型: Small Molecule
给药途径: Oral (PO)
适应症:
Tardive Dyskinesia(Approved);
Tourette's Syndrome(PhII);
Huntington's Disease(Development Outside U.S.).
推荐给药剂量:The initial dose for INGREZZA is 40 mg once daily. After one week, increase the dose to the recommended dose of 80 mg once daily. Continuation of 40 mg once daily may be considered for some patients. Administer INGREZZA orally with or without food
17. Brigatinib (Alunbrig)
药物名称: Brigatinib (Alunbrig)
公司: Ariad Pharmaceuticals
靶点:EGFR (Epidermal Growth Factor Receptor)
Anaplastic lymphoma kinase (ALK)
分子类型: Small Molecule
给药途径: Oral (PO)
适应症: ALK-positive NSCLC
推荐给药剂量: 90 mg orally once daily for the first 7 days; if 90 mg is tolerated during the first 7 days, increase the dose to 180 mg orally once daily
18. Abaloparatide (Tymlos)
药物名称: Abaloparatide (Tymlos)
公司: Radius Health
靶点: Parathyroid Hormone Receptor (PTHR)
分子类型: Protein
给药途径: Subcutaneous (SQ)
适应症: Osteoporosis / Osteopenia
推荐给药剂量: 80 mg subcutaneously once daily. Cumulative use of TYMLOS and parathyroid hormone analogs (e.g., teriparatide) for more than 2 years during a patient’s lifetime is not recommended. Patients should receive supplemental calcium and vitamin D if dietary intake is inadequate
19. Durvalumab (Imfinzi)
药物名称: Durvalumab (Imfinzi)
公司: AstraZeneca
靶点: Immune system Programmed death-1 receptor (PD-1) / Programmed death ligands (PD-L1 and PD-L2)
分子类型: Monoclonal Antibody
给药途径: Intravenous (IV)
适应症: Bladder Cancer(Approved);
Small Cell Lung Cancer (SCLC)(PhIII);
Non-Small Cell Lung Cancer (NSCLC) (PhIII);
Head and Neck Cancer(PhIII);
Melanoma(PhII);
Myelodysplastic Syndrome (MDS)(PhII);
Multiple Myeloma (MM)(PhII);
Hepatocellular (Liver) Cancer (HCC) (Including Secondary Metastases)(PhII);
Diffuse Large B-Cell Lymphoma (DLBCL) – NHL(PhII);
推荐给药剂量: 10 mg/kg administered as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity.
20. Sarilumab (Kevzara)
药物名称: Sarilumab (Kevzara)
公司: Sanofi
靶点: IL-6 Receptor (IL-6R)
分子类型: Monoclonal Antibody
给药途径: Subcutaneous (SQ)
适应症: Rheumatoid Arthritis (RA)(Approved)
Juvenile Rheumatoid Arthritis(PhII)
推荐给药剂量: 200 mg once every two weeks given as a subcutaneous injection. Reduce dose to 150 mg once every two weeks for management of neutropenia, thrombocytopenia and elevated liver enzymes
21. Edaravone (Radicava)
药物名称: Edaravone (Radicava)
公司: Mitsubishi Tanabe Pharma
靶点: Mitochondria
分子类型: Small Molecule
给药途径: Intravenous (IV)
适应症: Amyotrophic Lateral Sclerosis (ALS)
推荐给药剂量: an intravenous infusion of 60 mg administered over a 60-minute period according to the following schedule: An initial treatment cycle with daily dosing for 14 days, followed by a 14-day drug-free period. Subsequent treatment cycles with daily dosing for 10 days out of 14-day periods, followed by 14-day drug-free periods.
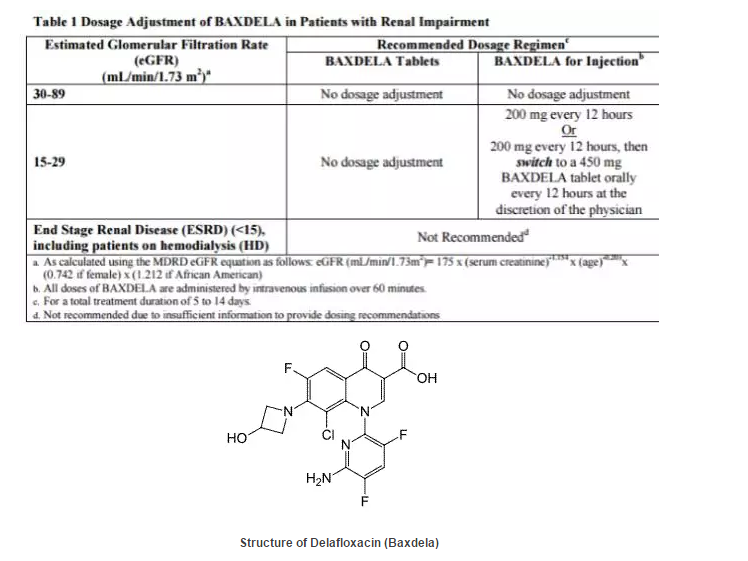
22. Delafloxacin (Baxdela)
药物名称: Delafloxacin (Baxdela)
公司: Melinta Therapeutics
靶点: Topoisomerase II (DNA gyrase) and IV
分子类型: Small Molecule
给药途径: Intravenous (IV), Oral (PO)
适应症: Skin and Skin-Structure Infections (Antibacterial)(Approved);
Community Acquired Pneumonia (CAP) (Antibacterial)(PhIII);
Hospital Acquired (Nosocomial) Pneumonia (HAP) (Antibacterial)(PhII).
推荐给药剂量:
23.Betrixaban (Bevyxxa)
药物名称: Betrixaban (Bevyxxa)
公司: Portola Pharmaceuticals
靶点: Coagulation Factor X
分子类型: Small Molecule
给药途径: Oral (PO)
适应症: Venous Thromboembolism (VTE)
推荐给药剂量: an initial single dose of 160 mg, followed by 80 mg once daily. Daily oral doses should be given at the same time of day with food. The recommended duration of treatment is 35 to 42 days.
